SAP IN ACCORDANCE WITH PHARMACEUTICAL LAW
All for One Poland offers a ready-made application that allows you to send messages to the Integrated System for the Monitoring of Trade in Medicinal Products (ZSMOPL) from the SAP system. Thanks to our solution, you can ensure compliance with the pharmaceutical law in Poland.
Our product is designed for SAP systems: S/4HANA Cloud Private; S/4HANA on-prem; SAP ECC/ERP version 6.0 or higher. If you use another SAP system or a system from another supplier, please ask us – we will offer an alternative solution.
Pursuant to provisions of the pharmaceutical law, the companies trading in medicinal products, foodstuffs intended for particular nutritional uses and medical devices are obliged to submit to ZSMOPL, on a daily basis, the data on stock transfers and levels, planned deliveries and reported shortages.
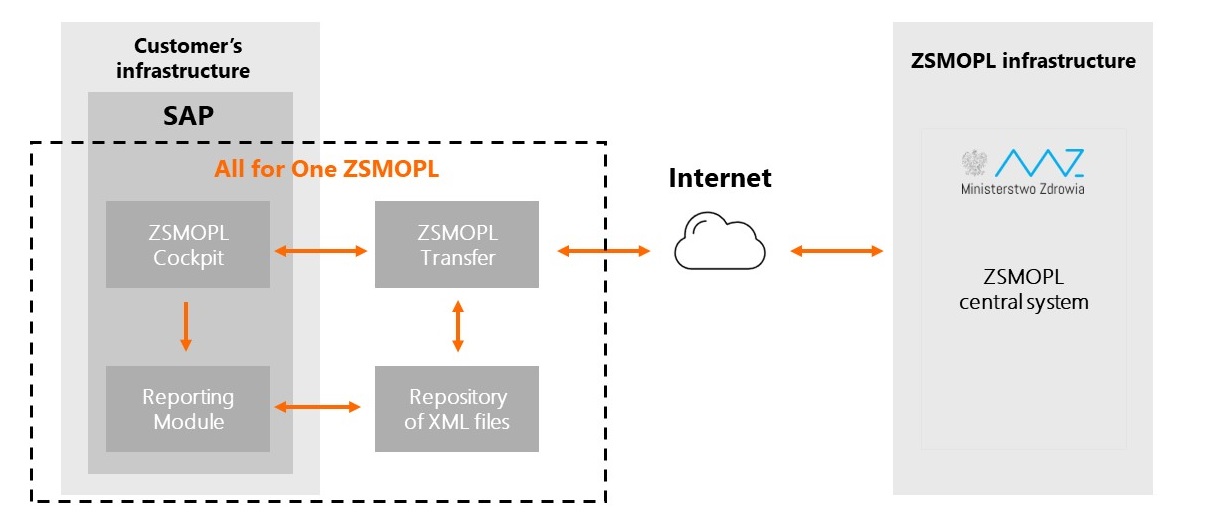
All for One ZSMOPL - solution architecture